   |
ORIGINS
AND HOST SPECIFICITY OF LEGUME-FEEDING PSYLLIDS (PSYLLOIDEA, HEMIPTERA)
IN THE MACARONESIAN ISLANDS
(Canary Islands and Madeira)
|
This
page is created and maintained by All images, unless otherwise noted, are copyright © Diana M. Percy
This
was my PhD (2001) research, and was undertaken at |
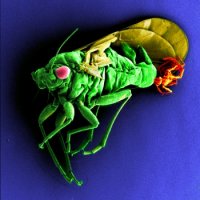
The legume-feeding psyllid, Arytinnis nigralineata (male), with a parasitic mite |
PhD thesis title: 'Diversification of legume-feeding psyllids (Psylloidea, Hemiptera) and their host plants (Genisteae, Leguminosae)'
PhD. Chapters available as PDF files: Chapter I, Chapter II; Chapter III; Chapter IV; Chapter V.
DATA MATRICES Morphological and Molecular appendices (anotated text and Nexus file formats).
Taxonomic diversity
This study focuses on psyllids that feed on legumes in the tribe Genisteae (Leguminosae), which includes the common broom, gorse and related shrubs (Percy 2002, 2003a, 2003b). The Genisteae is a Mediterranean centred tribe of papilionoid legumes comprising two main groups with associated genera (Bisby, 1981). Within the Canarian Genisteae, Genista, Retama and Teline are typically included in the Genista group, and Chamaecytisus and Spartocytisus in the Cytisus group, while Adenocarpus is an outlier. Recent phylogenetic studies of these genera include (Käss & Wink 1997; Percy & Cronk 2002). The genus Teline, totalling 13 species, 11 subspecies and two varieties, has undergone the greater part of this diversification in the Canary Islands. All Genisteae-feeding psyllids are in the tribe Arytaininae (Psyllidae) reviewed by Hodkinson and Hollis (1987).
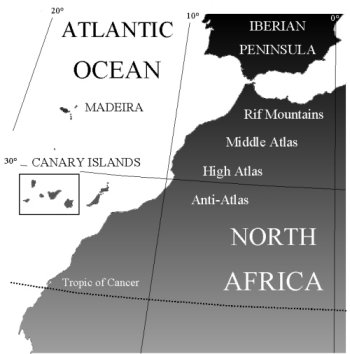
| The Macaronesian Islands The Macaronesian region showing the five archipelagos (north to south: Azores, Madeiras, Salvagens, Canaries, and Cape Verdes) which lie off the west coast of North Africa and southern Europe, between 15° and 40°N latitude. The geological ages of individual islands range from 1-30 Myr.
|
 |
| Africa and the Canaries As well as surveying the western Canary Islands and Madeira, I have looked for continental relatives of the Macaronesian psyllids from Genisteae hosts in North Africa, in particular the four montane regions - Anti-Atlas, High Atlas, Middle Atlas and Rif Mountains, and the Sierra Nevada in Southern Iberia. The Canaries incorporate 7 islands, the two eastern islands are closest to the African continent and are the oldest geologically - upto 21 Myr. These islands are relatively flat and dry with no native members of the legume or psyllid groups in this study. The 5 western islands range in age from 15 Myr for Gran Canaria in the east, to less than 1 Myr for El Hierro in the west - providing a chain of oldest to youngest island in an east to west direction. Distances between islands are in the region of 25-65 km, while Madeira is a considerable distance to the north. |
 |
The Western Canary Islands
The Canarian Genisteae is represented by six native genera (one endemic), and 17 species (Percy & Cronk 2002). The total number of native legume-feeding psyllids in the Canaries is 21 (Percy 2003a, 2003b). Numbers of psyllid and legume species are comparable for each island and there is greater diversity in both groups on higher and older islands.
| The Western Canary Islands with the number of psyllids and host plant group (Genisteae) for each island, and (in parenthesis) the number of species endemic to that island. Not all species of Genisteae are utilized as hosts, resulting in an uneven distribution of psyllids to host plants and frequent host sharing. |
 |
|
Habitat diversity: these images of the wet, laurel forest and dry, high subalpine zone (seen here with the endemic host genus Spartocytisus) give some idea of the diversity of habitats on a single island which may account for diversity in both psyllid and legume groups. |
|
| Although often monophagous (specific to a single host), there are widespread continental species complexes which occur on a few closely related hosts. When compared to the continent, psyllid diversity in the Canary Islands is extraordinarily high for such a small area. This is the largest Canarian psyllid species (right), about 4 mm in length, Livilla monospermae (male), on its host plant Retama monosperma. The host plants are in the papilionoid legume tribe Genisteae. |
 |
Speciation and host specificity
Strict cospeciation would predict a single psyllid species to be present on each legume species, and in fact the total number of psyllid species in the Canaries (21) is only marginally greater than the total number of Genisteae (17). When each island is taken separately, the number of psyllid and legume species are comparable, but as many as half the legume species may not be utilized as hosts. Plant and insect groups are subject to different species concepts and taxonomic treatments: a single plant species may appear to host many psyllids, but these may be associated with infraspecific host taxa (e.g. Teline stenopetala). In the Canaries, considerable infraspecific variety characterizes the plant classification and only one infraspecific taxon is recognized in the Canarian psyllid group.
Sympatric host sharing is common, but is mediated by ecological and temporal segregation which implies resource partitioning. On Teline microphylla, endemic to Gran Canaria, a widespread psyllid is sympatric in part of its range with one or more ecologically restricted species that are developmentally asynchronous with the widespread species. Similarly, the polymorphic legume, Teline stenopetala, is host on each island to two ecologically restricted species with partially overlapping ranges. Demand for spatial and nutrient resources are greatest during larval development when nymphs aggregate on developing plant organs, primarily new leaves, flower buds, and young fruit. By avoiding temporal competition during seasonal plant growth, more than one psyllid species can utilize similar resources.
The specificity of psyllids on Adenocarpus hosts on Tenerife may partly be maintained by competetive exclusion. On the two high islands, Tenerife and La Palma, the plant genus Adenocarpus has diversified into a lowland and an upland species. On Tenerife, each Adenocarpus species has a host specific psyllid (and both species occur in a mid-altitude hybrid zone) but on La Palma, only one psyllid appears to have colonized this island, where it can be found on both lowland and upland hosts. Transplant experiments in the field have indicated that both psyllids will accept either host. One likely explanation of how host specificty is maintained in Tenerife (as an apparent result of competetive exclusion in this case) is the effect of host phenology: Adenocarpus foliolosus, which occurs at lower altitudes and in wetter habitats, flowers earlier than Adenocarpus viscosus.
| Flower phenology and egg laying preferences of Arytinnis proboscidea on Adenocarpus viscosus Adenocarpus inflorescences are racemes composed of mature flowers at the base of the raceme with progressively younger flowers towards the tip. I found the majority of eggs on flowers that had the corolla just emerging from the calyx (as seen in the photograph), indicating that preference in females to oviposit on flowers at a particular stage of development may be important in maintaining host specificity. |
 |
The main questions I addressed:
1. Are the Genisteae-feeding psyllids monophyletic?
2. What is the taxonomic status of the psyllid genus Arytainilla into which all but one of the Canarian psyllids had been placed (see Percy 2003a )?
3. How many colonizations account for the present psyllid diversity in the Canary Islands?
4. Can preadaptation in a potential colonizer to available host plants explain the pattern of host specificity in the Canaries and Madeira?
5. Is there critical host plant density, required to support a specific psyllid fauna?
This last question, concerning host abundance, together with infraspecific host variation and host hybidization appear to be important factors influencing host specificity.
Anthropogenic influences
Man can critically affect the genetic diversity and geographic range of hosts through habitat disturbance, and by host cultivation. This can lead to the contraction of some host ranges while expanding others. Indirect host expansion (through competetive invasion of disturbed habitats and hybrid zones) and direct expansion by cultivation, can both result in high abundance of associated psyllid species. Man’s influence is perhaps more visible on the legume hosts than their insect faunas, but the fate of the host is closely linked - probably ‘tracked’ by the psyllids.
| On La Palma, the grazing of goats has reduced the two legume species native to the subalpine zone, and once thought to be widespread, to threatened status, and neither has a psyllid fauna. Only six adult plants of Genista benehoavensis were recorded in 1988. Now there is a successful regeneration program underway (photograph), but this area is currently dominated by a near monotypic invasion of the less palatable legume, Adenocarpus viscosus, accompanied by a parallel explosion of its psyllid fauna. |  |
| A more direct influence of man in promoting the abundance of both host and psyllid fauna can be found in the common cultivation of the native legume, Chamaecytisus proliferus, as a fodder crop - in the photograph below are a large number of psyllids specific to this host - which have been collected from a single wild individual. |  |
Host plant hybridization
For many insular island groups, species radiations are geographically and ecologically maintained, and morphological diversity is not matched by genetic diversity. Where environmental barriers break down, hybridization and introgression result (Humphries, 1976; Lems, 1958). Although vastly accelerated in recent times, there is evidence from the complex hybrid zone in the Guïmar region, that early settlement by the Guanches in this area promoted hybrid habitats for plants in a number of endemic genera. Host hybridization appears to be important for psyllid diversity. Plant hybridization may facilitate novel host colonization and the diversification or expansion of psyllid host preference. Two examples have correlated host acquisition by psyllids and host plant hybridization without involving a switch from the original host (Arytinnis pileolata and Arytinnis equitans).
A critical host abundance?
The Genisteae species on which no psyllids have been found, survive in relict populations (< 2000 individuals). The survival of a host specific psyllid is likely to be affected not only by total host abundance, but by fragmentation of host populations.
Preadaptation for host preference
Comparing host associations in continental source areas with those in the Canaries and Madeira provides an insight into preadaptation for host preference, and changes in host association resulting from island colonization, radiation and ecological specialization. Psyllid genera in the Canaries are specific to the same host genera, or generic complex, as their closest continental relatives. Thus in all cases, successful colonization appears to be restricted by, and dependent on, preadaptation to available hosts.
Host phylogeny
The systematics of the legume group are far more well studied than the psyllid group.The most recent molecular phylogeny for the host plant group is by Käss & Wink (1997) and Percy & Cronk (2002). The monophyly of the Genisteae sensu stricto is confirmed using ITS regions 1 and 2 and the rbcL gene. However, groupings within the Genisteae are still somewhat ambiguous, and the taxonomic history of this group has been confusing, with nearly every member of the Canarian Genisteae having been classified under both Genista and Cytisus at one time or another (Percy & Cronk 2002).
Island colonization - molecular evidence
| I have sequenced two mitochondrial regions for the psyllid group. One region of 350 bp of the small subunit rRNA, (or 12S), and a second region of 612 bp incorporating the 3’ end of COI (252 bp), the tRNA leucine (66 bp) and the 5’ end of COII (283 bp), making a combined total of 962 bp. | 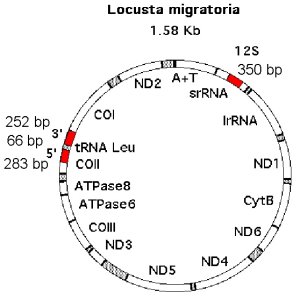 |
| 12S (small subunit rRNA) I have sequenced the 12S region for 61 psyllid taxa. 17 of these are new species (Percy 2002, 2003a, 2003b). Included are the five genera known to feed on host plants in the Genisteae: Arytaina predominantly on hosts in the Cytisus group, Arytainilla on all three host groups, Arytinnis and Livilla predominantly on hosts in the Genista group, and Pseudacanthopsylla, comprising two species on Retama in the Genista group. The outgroup species are from other groups in the family Psyllidae, including Acizzia from mimosoid legume hosts and Cacopsylla from hosts in the Rosaceae and Rhamnaceae. The latter is typically considered to be the sister group to the Genisteae feeders. AI = Arytinnis; AR = Arytaina; L = main Livilla group. Thick branches indicate bootstrap support greater than 75% in this maximum parsimony analysis. |
 |
Only two groups are well resolved, the genus Arytaina, and the predominantly Macaronesian genus, Arytinnis. The paraphyly of Arytainilla (into which all but one of the Canarian species had been placed at one time) is apparent.
The main groups are:
1. the genus Arytaina, including three Canary Island species
2. the mostly Macaronesian group, predominantly on hosts in the Genista group, and described as a new genus, Arytinnis (Percy 2003a )
3. Arytainilla sensu stricto, predominantly on Cytisus group hosts
4. the main Livilla group, predominantly on hosts in the Genista group (this does not include the type species group)
5. a small Livilla group, mainly on the genus Adenocarpus
6. Pseudacanthopsylla - the placement of this genus is not fully resolved.
Interestingly, the molecular phylogeny shows that the placement of taxa is often better predicted by the host plant, than by the current taxonomic classification. Such that the main psyllid groups above are characterized by similar host associations, and in the genus Livilla, there is a group predominantly on Genista species, the Retama-feeding species, and the species on Adenocarpus.
There has been a minimum of four, possibly five colonizations of the Canary Islands. One of these has resulted in a radiation of psyllids predominantly on the host genus Teline. Four species at the base of the Canarian group occur on or are endemic to Gran Canaria, making this island the most likely place for the initial diversification of the group, followed by colonization of the other islands, a single dispersal to Madeira and possibly two colonizations of the continent. Lack of resolution on the backbone of the tree does not provide confidence in how the different Arytaininae groups are related to each other or to the other Genisteae-feeding genera. The monophyly of the Genisteae-feeding species is not strongly supported (72%) but the same topology is recovered in the strict MP and ML trees.
| COI, tRNA Leu and COII The cytochrome oxidase region provides aproximately double the amount of genetic variation found in the 12S region, and combining the 12S and CO data produces a fairly robust phylogeny. A = Arytainilla sensu stricto; AI = Arytinnis; AR = Arytaina; L = main Livilla group. Thick branches indicate bootstrap support greater than 75% in this maximum parsimony analysis. |
 |
So far I have mainly used cytochrome oxidase to look at inter-island colonizations between and within species in the Canary Islands. There is considerable variation between, in some cases, populations on different islands (in the species Arytaina devia and Arytinnis dividens) and populations on different hosts (in the species Arytinnis pileolata and Arytinnis diluta). The lack of variation detected in populations on different hosts may imply that there is significant geneflow between these populations, or alternatively, that the acquisition of new hosts is a recent phenomenon, e.g. via host hybridization.
In the genus Arytaina, infraspecific genetic variation between island populations (in Arytaina devia) is contrasted with much less variation in widespread continental species (in Arytaina genistae), implying there may be significant barriers to gene flow between islands. Incidentally, Arytaina devia occurs on the same host on all four islands whereas Arytaina genistae occurs on several closely related Cytisus species throughout its continental range. Interpreting the direction of island colonization is also possible in Arytaina devia, Arytinnis dividens.
| Mapping host associations onto the molecular tree There is little congruence between host and psyllid phylogenies, except in some instances, at the tips of the trees. But major associations and constraints are evident, in this case to hosts in either the Cytisus group (in yellow) or Genista group (in blue), or the host genus Adenocarpus (in red). So that when host associations in these three host categories are mapped onto the 12S tree here, it becomes apparent that not cospeciation but preadaptation to closely related hosts determines successful colonization of host plants in the Canaries (Canarian species are indicated by the bars and arrows), with subsequent host switching occurring in at least two instances in the Canaries. |
Cladogram of legume-feeding psyllids with host associations mapped as an unordered character. |
| To summarise the pattern of host usage in the Canaries: The majority of psyllids (14) are specific to a single host, while the remaining (6) occur on two hosts and one species occurs on three hosts. In cases where a psyllid has more than one host, hybridization between hosts may have facilitated the acquisition of multiple hosts. Where it appears that many psyllid species occur on the same host, a number of these are specific locally in only part of the host range or to infraspecific taxa on different islands. This figure shows Canarian psyllid species with maximum number of hosts and psyllid distributions on host species (C = Gran Canaria; T = Tenerife; G = Gomera; P = La Palma; H = El Hierro). |
 |
Summary
The dynamics of psyllid speciation and diversity in the Canary Islands are closely linked to local host plant ecology. Host plant abundance, population structure, phenotype and hybridization may all be important in determining host specificity. Preadapted host preference, as indicated by historical host associations in continental progenitors, explains generic host plant selection and may also account for the uneven distribution of psyllids on available hosts in the genus Teline. Understanding processes that result in the sharing of a single host, both allopatrically and sympatrically, by two or more psyllid species is thought to be key in evaluating host recognition and selection. Habitat destruction endangers both host plant and psyllid species. Absence of psyllids on rare legumes may imply that a critical host plant density is required to support a host specific psyllid fauna.
I conclude that host specificity in the Canary Islands is determined by four main components:
1. Preadaptation - in potential colonizers to hosts that are closely related to the host from the source area.
2. Local adaptation - that may be mediated by climate or host physiology and ecology.
3. Host abundance - there may be a critical host abundance below which a specific psyllid can not be supported.
4. Host hybridization - habitat disturbance may have promoted hybrid habitats and hydridization in hosts with subsequent expansion of host preference in psyllids.
| Further Work Host transplant experiments will be used to test psyllid specificity between infra- and interspecific hosts and between host genera. |
 |
Morphology (psyllids)
Matrix of 67 morphological characters and character states. Inapplicable or missing characters are indicated by '-' and '?' respectively. Nexus format. Excel document. Text document.
List of 67 morphological characters and character states. Word document.
See also Chapter III of my PhD thesis available in PDF format
Molecular (psyllids) (two mitochondrial regions were sampled)
12S (small subunit ribisomal rRNA), aligned matrix is 342bp. Nexus format. Word document. Text document.
COI + tRNA Leu + COII (cytochrome oxidase I, tRNA leucine, cytochrome oxidase II), aligned matrix is 639bp. Nexus format. Word document. Text document.
See also Chapter III of my PhD thesis available in PDF format
Molecular (legumes)
ITS1-5.8S-ITS2 (internal transcribed spacers and 5.8S region of nuclear ribosomal DNA), aligned matrix is 637bp. Nexus format. Word document. Text document.
See also Chapter IV of my PhD thesis available in PDF format
Acknowledgments
This work forms part of a doctoral thesis at the University of Glasgow, funded by the Natural Environment Research Council (NERC) and supervised by Rod Page (Glasgow) and Quentin Cronk (Edinburgh; now at UBC, Canada). Grants were received from the Carnegie Trust for the Universities of Scotland, and the Louise Hiom award (Glasgow University) which enabled me to undertake field work in the Moroccan Atlas Mountains.
References
Bisby, F. A. (1981). Genisteae (Adans.) Benth. (1865). In Advances in Legume Sytematics (ed. R. M. Polhill and P. H. Raven), pp. 409-425. Kew, Royal Botanic Gardens.
Greinwald, R., Bachmann, P., Witte, L., Acebes-Grinoves, J.R. & Czygan, F-C. (1992). Taxonomic significance of alkaloids in the genus Adenocarpus (Fabaceae-Genisteae). Biochem. Syst. Ecol. 20, 69-73.
Hodkinson, I. D. (1980). Present-day distribution patterns of the holarctic Psylloidea (Homoptera: Insecta) with particular reference to the origin of the nearctic fauna. J. Biogeog. 7, 127-146.
Hodkinson, I. D. & Hollis, D. (1987). The legume feeding psyllids (Homoptera) of the west Palaearctic Region. Bull. Br. Mus. nat. Hist. (Ent.) 56, 1-86.
Humphries, C. J. (1976). Evolution and endemism in Argyranthemum Webb ex Schultz Bip. (Compositae: Anthemideae). Botanica Macaronesia 1, 25-50.
Janzen, D. H. (1979). New horizons in the biology of plant defences. In Herbivores. Their Interaction with Secondary Plant Metabolites. (ed. G. A. Rosenthal and D. H. Janzen), pp. 331-350. Academic Press, New York.
Jermy, T. (1976). Insect-host-plant relationship - coevolution or sequential evolution? Symposia Biologica Hungarica 16, 109-113.
Käss, E. & Wink, M. (1997). Phylogenetic relationships in the papilionoideae (family leguminosae) based on nucleotide sequences of cpDNA (rbcL) and ncDNA (ITS 1 and 2). Molecular Phylogenetics and Evolution 8, 65-88.
Lems, K. (1958). Botanical notes on the Canary Islands I. Introgression among the species of Adenocarpus, and their role in the vegetation of the Islands. Bol. Inst. Nac. Invest. Agron. Madrid 39, 351-370.
Menken, S. B. J. (1996). Pattern and process in the evolution of insect -plant associations: Yponomeuta as an example. Entomologia Experimentalis et Applicata 80, 297-305.
Mitter, C., Farrell, B. & Wiegmann, B. (1988). The phylogenetic study of adaptive zones: has phytophagy promoted insect diversification? The American Naturalist 132, 107-128.
Percy, D.M., Page, R.D.M. & Cronk, Q.C.B. (2004) Plant-insect interactions: double-dating associated insect and plant lineages reveals asynchronous radiations. [Invited paper as part of the ‘Untangling Coevolution’ symposium held at the 2001 Evolution meeting, Illinois]. Systematic Biology 53, 120-127. pdf (233 kb)
Percy, D.M. (2003a) Legume-feeding psyllids (Hemiptera, Psylloidea) of the Canary Islands and Madeira. Journal of Natural History 37, 397-461. pdf (1980 kb)
Percy, D.M. (2003b) Radiation, diversity and host plant interactions among island and continental legume-feeding psyllids. Evolution 57, 2540-2556. pdf (1599 kb)
Percy, D. M. (2002) Distribution patterns and taxonomy of some legume-feeding psyllids (Hemiptera; Psylloidea) and their hosts from the Iberian Peninsula, Morocco and Macaronesia. Journal of Insect Systematics and Evolution 33, 291-310. pdf (1054 kb)
Percy, D. M. & Cronk, Q. C. B. (2002). Different fates of island brooms: contrasting evolution in Adenocarpus, Genista and Teline (Genisteae, Leguminosae) in the Canary Islands and Madeira. American Journal of Botany 89, 854-864. pdf (207 kb)
Thompson, J. N. (1994). The Coevolutionary Process. University of Chicago Press, Chicago.
Wink, M. (1992). The role of quinolizidine alkaloids in plant-insect interactions. In Insect-Plant Interactions, vol. 4 (ed. E. Bernays). CRC Press, Boca Raton.
Go to: psyllid Home page, psyllid acoustics, psyllid morphology, Macaronesian island psyllids, Pacific island psyllids, psyllids of economic importance
All images, unless otherwise noted, are copyright © Diana M. Percy
Created 18/01/2000. Updated 20/01/2005. Return to top of page
psyllids of economic importance
psyllid taxonomy, host plants, and bibliography site by David Ouvrard et al